Post 28: The protein folding 🔥
Published:
“Vibras y vibras tan lindo. Y vibramos juntos, entre
We can imagine proteins as parts of water and oil. The oil parts (hydrophobic amino acids) will seek to be together in the center, while the water parts (non-hydrophobic amino acids) will tend to be on the surface. The interaction between these parts determines a certain energy state in which the protein adopts a folding. And for a protein to function within the cell, it usually adopts a condition of minimum energy.
Therefore, a protein is actually a multitude of energy states (or conformations), and only a few of them are capable of carrying out its function. If we study these conformations in detail, we can notice that some will have a greater or lesser probability of being inhibited by x molecule and thus learn more about its function and design drugs.
This is what the video illustrates, on the left the protein folding and on the right a map of its energy:
And if you want to know more about it, this nice and VERY complete review:
And how is that energy calculated? Well, in reality, no one knows in detail, but the best approximation we have is the “energy functions” that consider different physicochemical, statistical, and geometric parameters of proteins. Perhaps, the energy function of the program called “Rosetta” is the most popular.
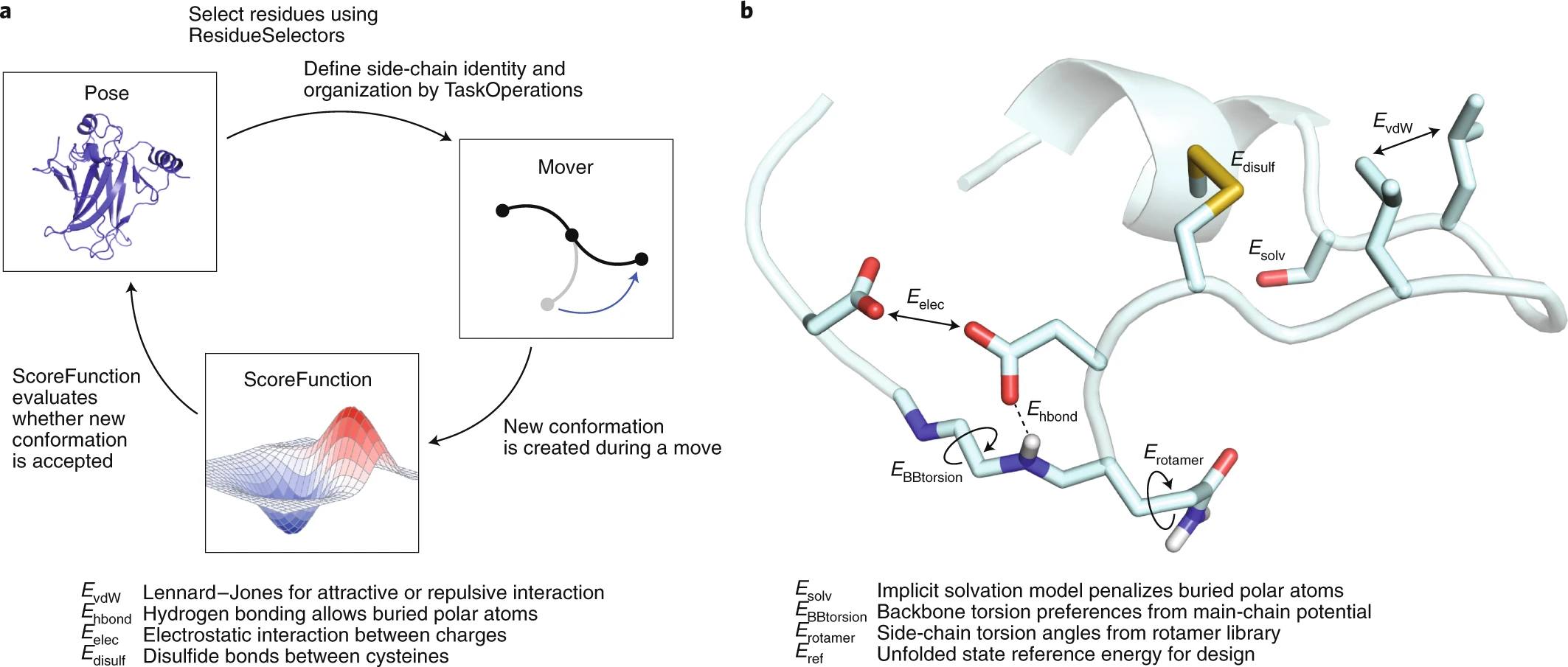
Ref:
